Describe the Relationship Among Temperature Heat and Thermal Energy
The molecules in a substance have a range of kinetic energies because they dont all move at the same speed. As heat is introduced into a system the molecules absorb the thermal energy and increase their kinetic energy.
Difference Between Temperature And Thermal Energy Difference Between
Temperature is a measure unit of the average kinetic energy in a system.

. Thermal energy is the total energy the body has due to movement of inner molecules and bonds between them and heat is the change in. When we heat a substance we use some energy generally from some fuel. Heat is the transfer of thermal energy whereas temperature is a property the object exhibits.
Heat is a product of thermal energy. Temperature is the amount of heat in degrees the object has. It is the energy transferred from one system to another that is not done by work.
As such when heat is in transit you have thermal energy. Heat and temperature are a closely related topic and as such the difference between the two can be a bit confusing. What is the relationship between heat temperature and thermal energy within a system.
Temperature degree or intensity of heat present in a substance or object especially as expressed. In other words it is a heat presented in degrees and it is proportional to it. When temperature will increase particles in an object speed up which implies a rise in thermal energy.
Describe the relationship among temperature heat and thermal energy. Temperature heat and thermal energy are parts of one system. 359 Views Describe the relationship among tempreture heat and thermal energy Describe the relationship among tempreture heat and thermal energy Answer they all produce energypower they are alike because they do the same thing just in different ways To see more answers head over to College Study Guides Virtual Teaching Assistant.
Heat is the transfer of thermal energy between objects that have different temperatures. Thermal Energy and Heat. How much heat energy is lost by 3 kg of water when it cools from 80 degrees C to 10 degrees C.
Energy can neither be created nor destroyed but transferred to a lesser form. Thermal heat is what is given off when energy is lost. Heat and temperature are different but very closely related heat a flow of energy from an object at a higher temperature to an object at a lower temperature thermal energy the total random kinetic energy of particles of an object energy flows from warmer to cooler objects.
Because according to the Law of Conservation of Energy. Define heat and temperature and convert between temperature scales. Answer 1 of 7.
However when it reaches this temperature applying more increasing thermal energy does not cause the temperature to rise. Among the temperature thermal energy and thermal energy transfer ie. There you have it the difference between heat and thermal energy.
Thermal energy always moves from an object with a higher temperature to an object with a lower temperature. During a phase change the temperature remains mainly constant. Heat is energy in transport and it is not an equation of state.
Temperature is in fact a measure of the kinetic energy of molecules. What is the relationship between thermal enery and heat. Heat is what is given of from thermal energy.
Heat is not a property of a system. The core difference is that heat deals with thermal energy whereas temperature is more concerned with molecular kinetic energy. Since the particles ar moving additional now the potential energy.
Thermal energy is what is given off when energy is lost. Thermal energy tells us how much transfer of energy is caused by the temperature differences in two objects. While thermal energy refers to the total energy of all the molecules within the object heat is the amount of energy flowing from one body to another spontaneously due to their temperature difference.
This is because atoms and molecules are moving around bumping into one another. Heat is the total energy of the motion of the molecules inside the object or particle whereas Temperature is merely a measure of this energyThe relationship could be the more heated an object is there higher the temperature the object will have. As a subtance absorbs heat the particles move faster so the average kinetic energy and.
What are the relationships among temperature heat and thermal energy. Temperature is the amount of heat an object has. When you apply increasing thermal energy to a certain material it reaches a temperature of 50 degrees C.
Specific heat is the amount of energy in joules needed to raise the temperature o. This indicates how strong in your memory this concept is. Heat in the kinetic molecular theory and apply those representations to qualitatively and quantitatively describe how changing the temperature of a substance affects the motion of the molecules.
Heat is what is given off from thermal energy. Heat Temperature and Phase Changes Assessment Practice Answer This graph shows what happens to the temperature of the water while it was boiling in a kettle as heat energy was being added to it and what the water was doing will it began to warm and change states. Find step-by-step Physical science solutions and your answer to the following textbook question.
Practice Heat Temperature and Thermal Energy Transfer. Difference between heat temperature and thermal energy - sevonte energy Difference between heat temperature and thermal energy Thermal energy. Correct answer to the question Describe the relationship among heat temperature and thermal energy.
Thermal energy is an equation of state it is the total energy contained in a system due to the random motions of all of its constituents in the center of mass frame. Heat is a form of energy but it is energy in transit.
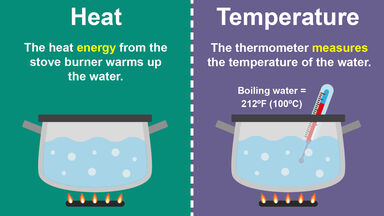
Difference Between Heat And Temperature In Simple Terms
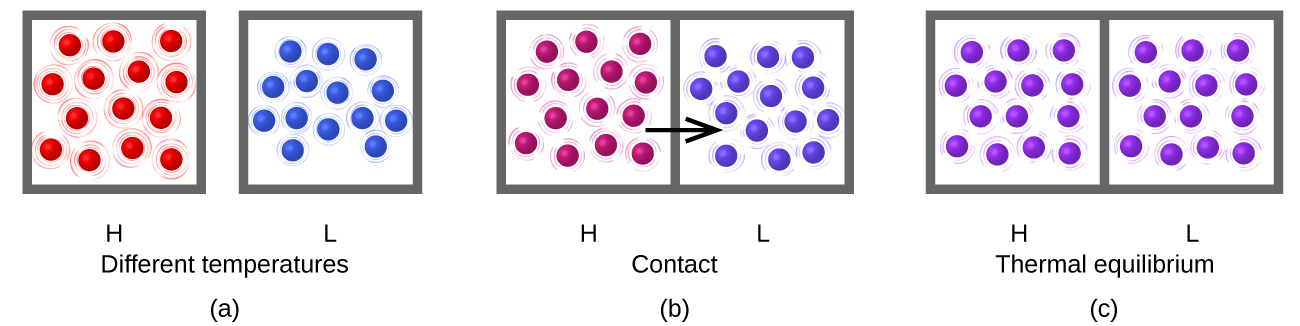
7 8 Quantifying Heat Chemistry Libretexts

Temperature Thermal Energy And Particle Motion Science Games Legends Of Learning
Difference Between Temperature And Thermal Energy Difference Between

Heat Temperature And Thermal Energy Transfer Read Physics Ck 12 Foundation
Difference Between Temperature And Thermal Energy Difference Between

Difference Between Heat And Temperature Comparison Measurement

What S The Difference Between Temperature And Heat Albert Io

What Is Thermal Energy Article Khan Academy

What Is The Difference Between Heat And Temperature Thermodynamics Physics Youtube

Heat Vs Temperature What Are The Similarities Differences W Graph

Thermal Energy Heat And Temperature Youtube

What Is The Difference Between Thermal Energy And Heat Lisbdnet Com
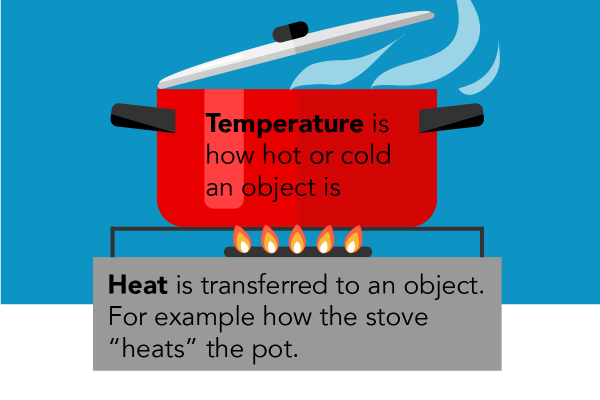
Introduction To Heat Transfer Let S Talk Science
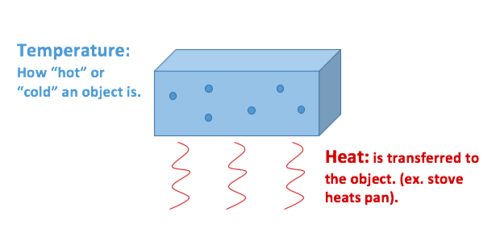
Heat Vs Temperature Energy Education
Thermal Energy Vs Heat Thermodynamics Psiberg


Comments
Post a Comment